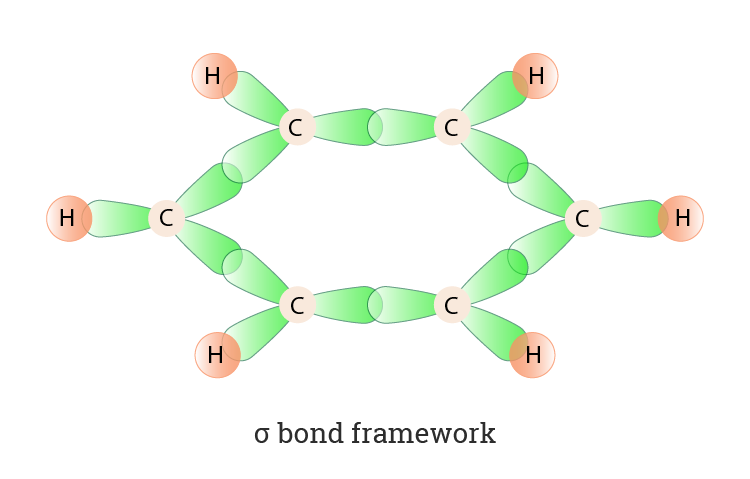
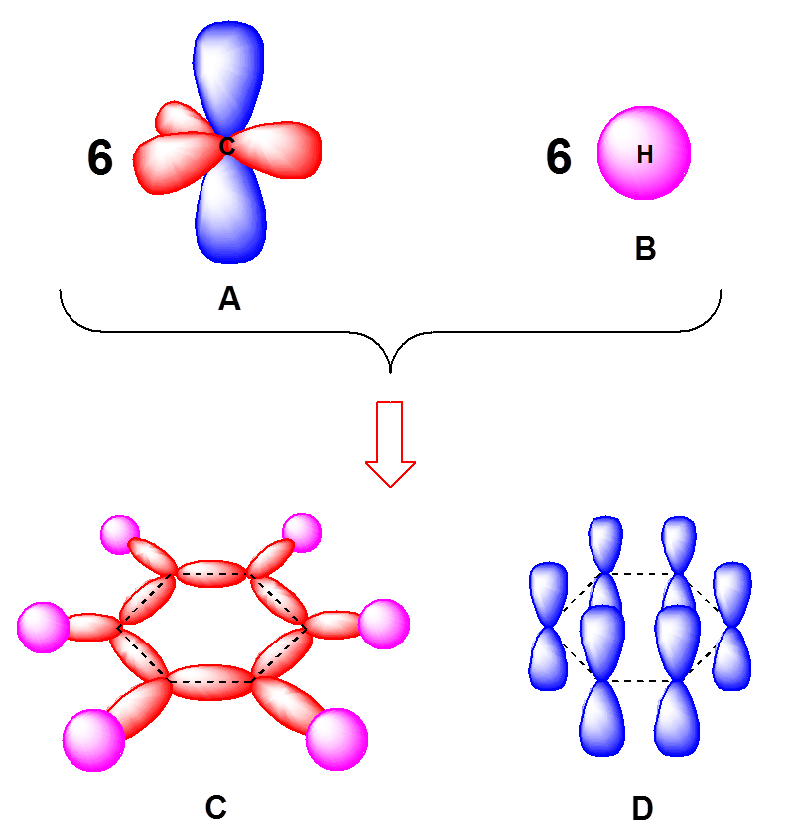
Why does benzene form sp2 hybridisation and not sp3? Why does one zth 2p orbital not participate in hybridization? Can someone explain this briefly? - Quora

SOLVED: 1. a) Label the hybridization of each carbon atom in benzene Next draw 3-D representation of benzene clearly showing all pi orbitals (including electrons) How does this demonstrate that the pi

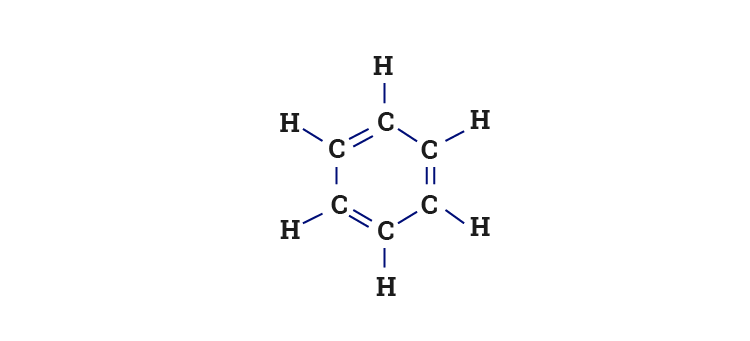
What is the hybridization of each carbon atom in benzene? What shape do you expect benzene to have? | Homework.Study.com

Benzene Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram - Techiescientist
Why does benzene form sp2 hybridisation and not sp3? Why does one zth 2p orbital not participate in hybridization? Can someone explain this briefly? - Quora
Why does benzene form sp2 hybridisation and not sp3? Why does one zth 2p orbital not participate in hybridization? Can someone explain this briefly? - Quora
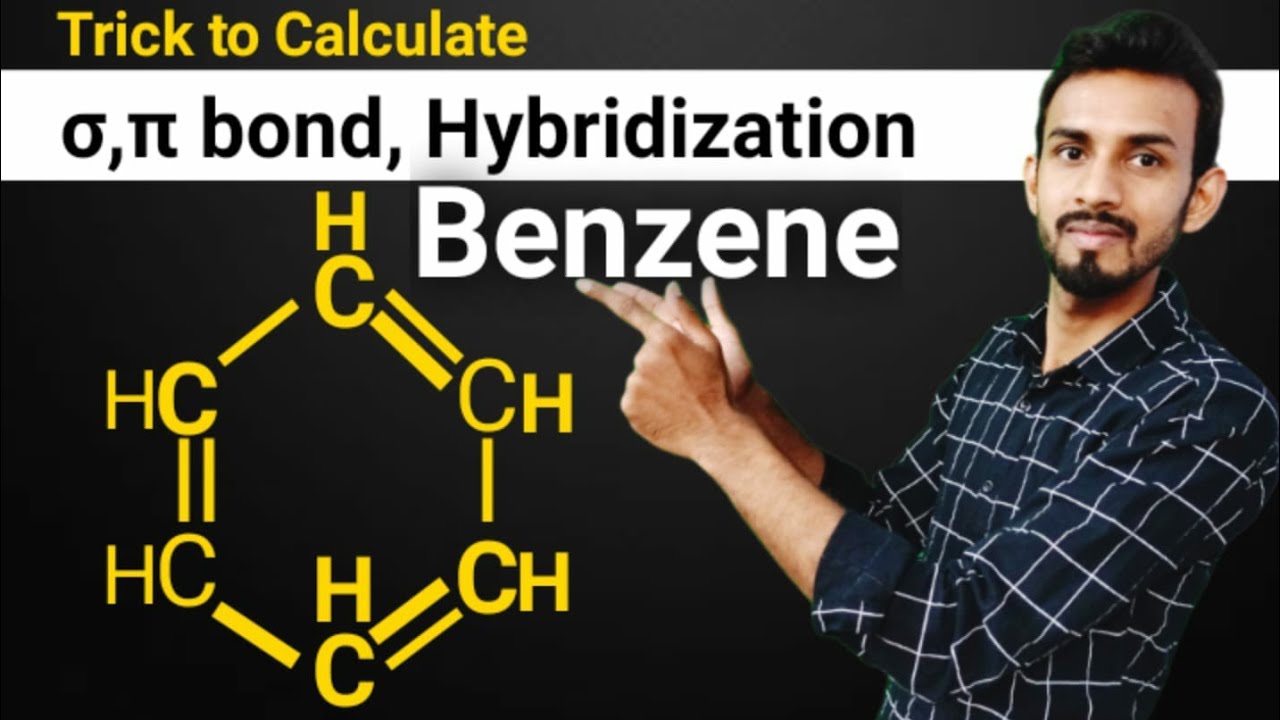

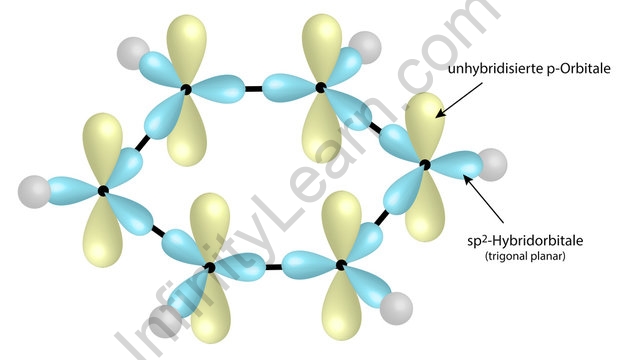



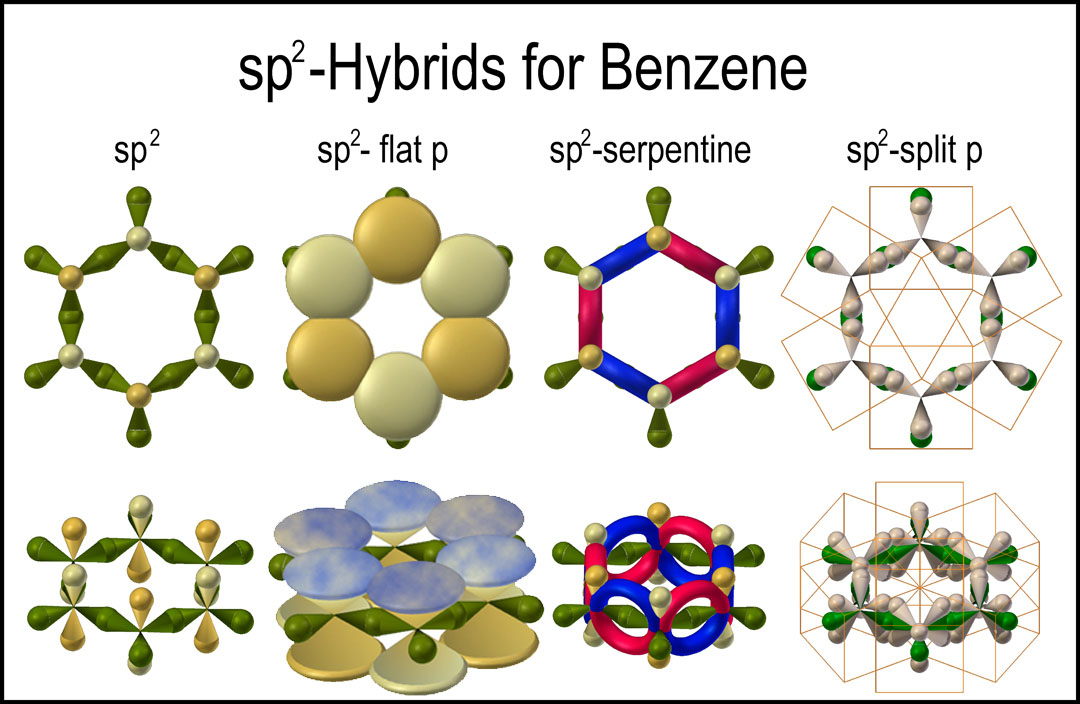
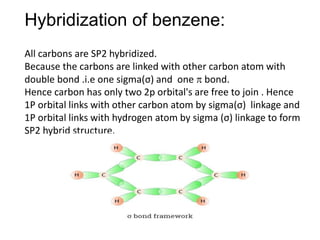

![The number of \\[s{p^2}\\] hybrid orbitals in a molecule of benzene is:A.12B.24C.18D.6 The number of \\[s{p^2}\\] hybrid orbitals in a molecule of benzene is:A.12B.24C.18D.6](https://www.vedantu.com/question-sets/65ee942c-4efe-46c3-8357-31e88b50e8f63177530697027051495.png)