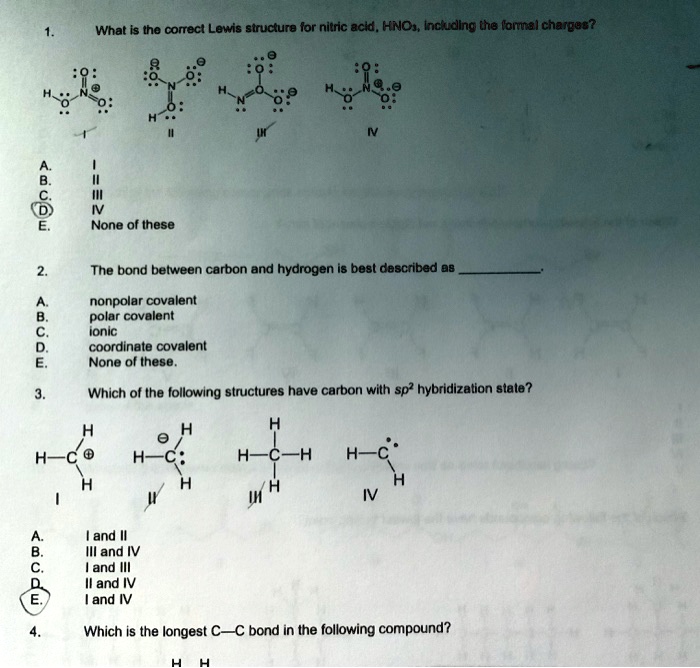
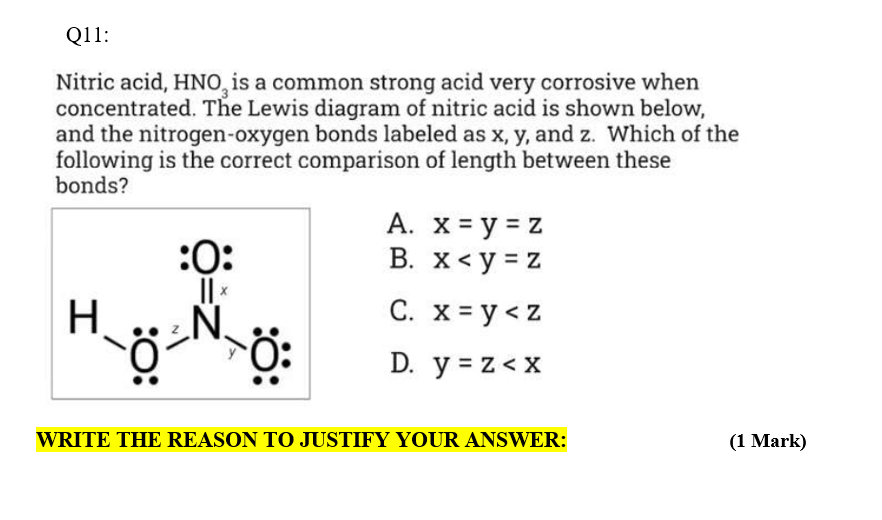
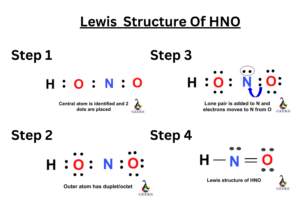
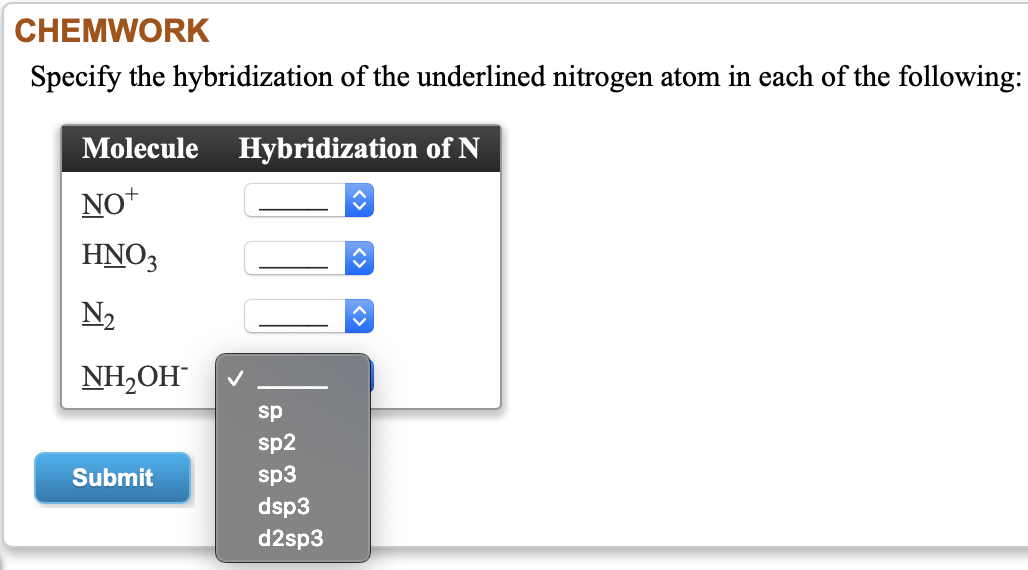
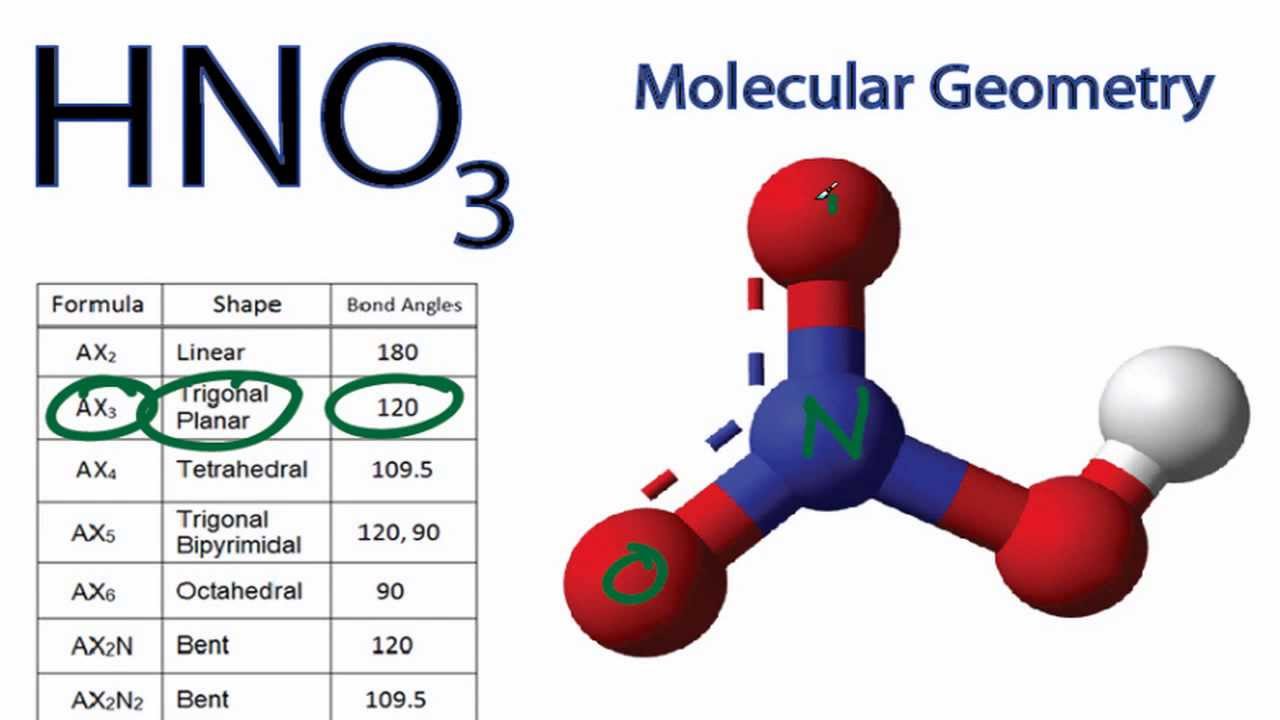
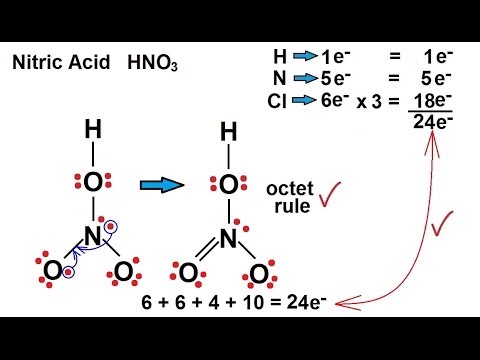
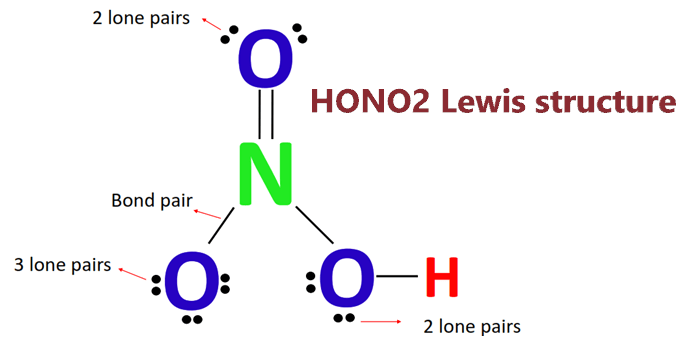
Provide the following information for the compound HNO3. a. Lewis dot structure b. hybridization c. electron geometry d. molecular geometry e. polarity | Homework.Study.com

What is nitric acid (HNO3) in chemistry? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
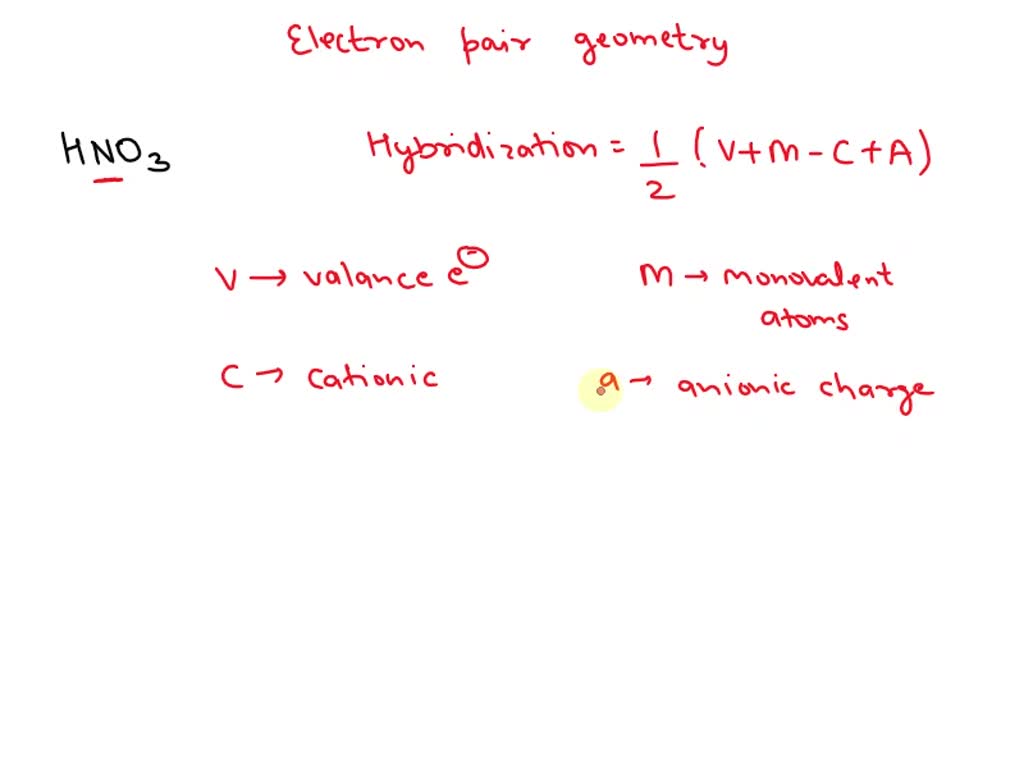
SOLVED: What is the electron-pair geometry about the nitrogen atom in the HNO3 (nitric acid) molecule. Hint: The nitrogen atom is the central atom and the hydrogen atom is attached to an

Nitric acid, HNO_3, dissociates in water to form nitrate ions and hydronium ions. What change in the hybridization of the nitrogen atom occurs in this dissociation? (a) sp_2 to sp_3 (b) sp_2