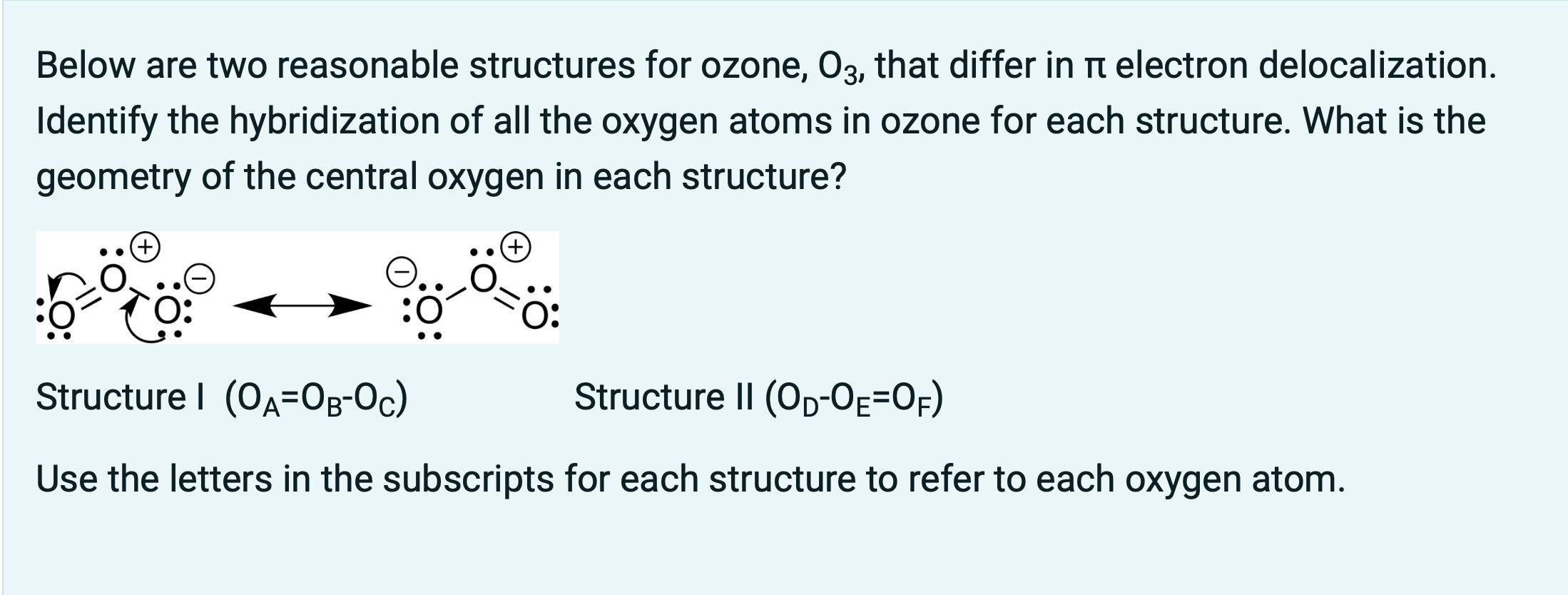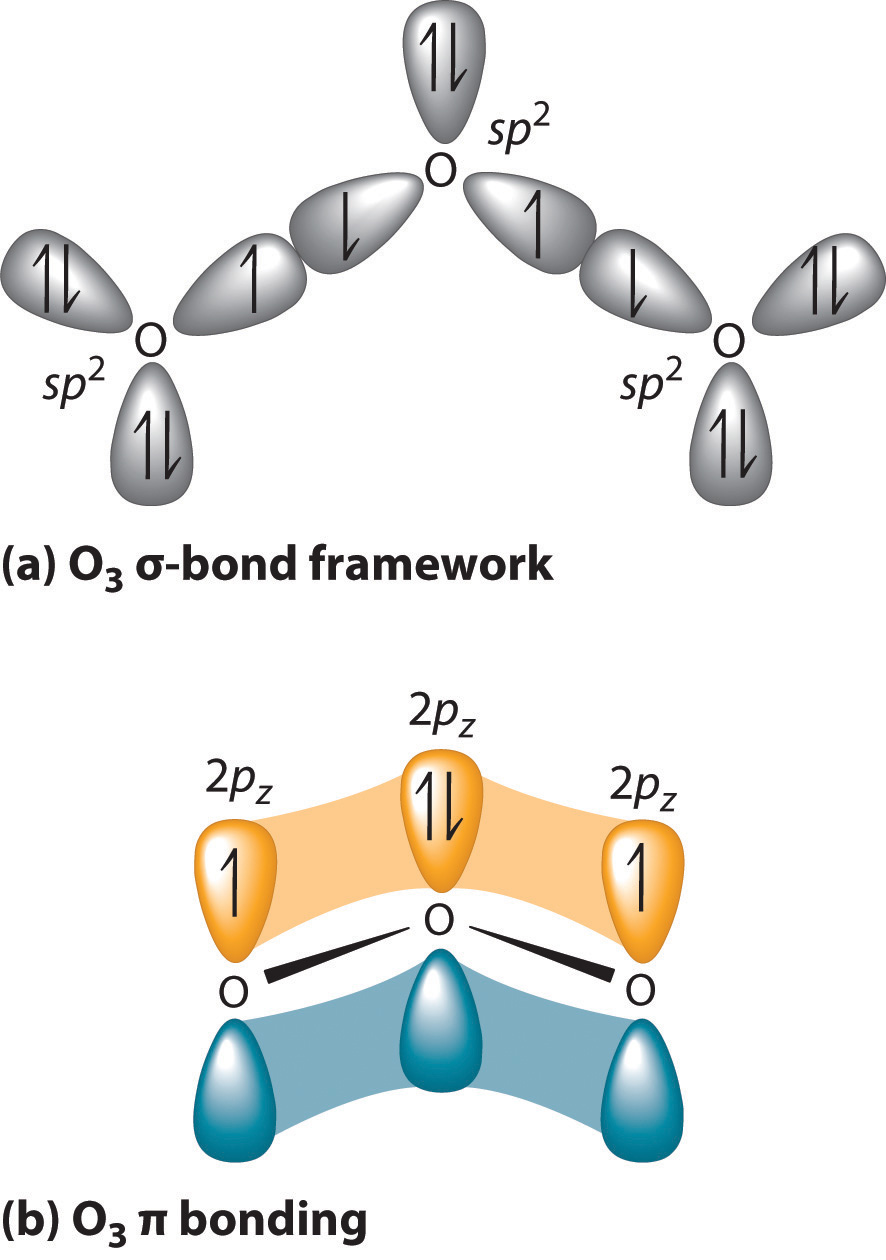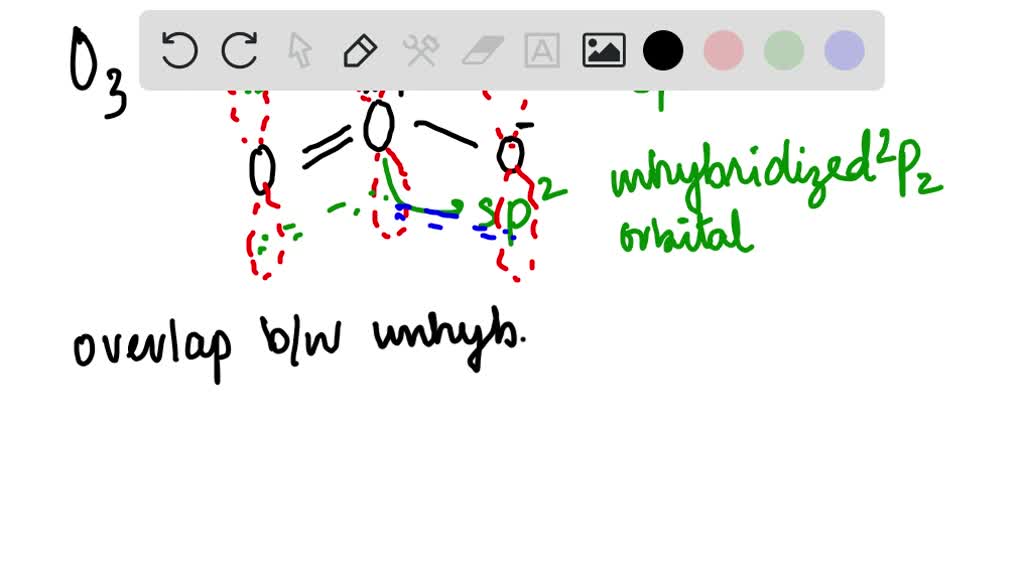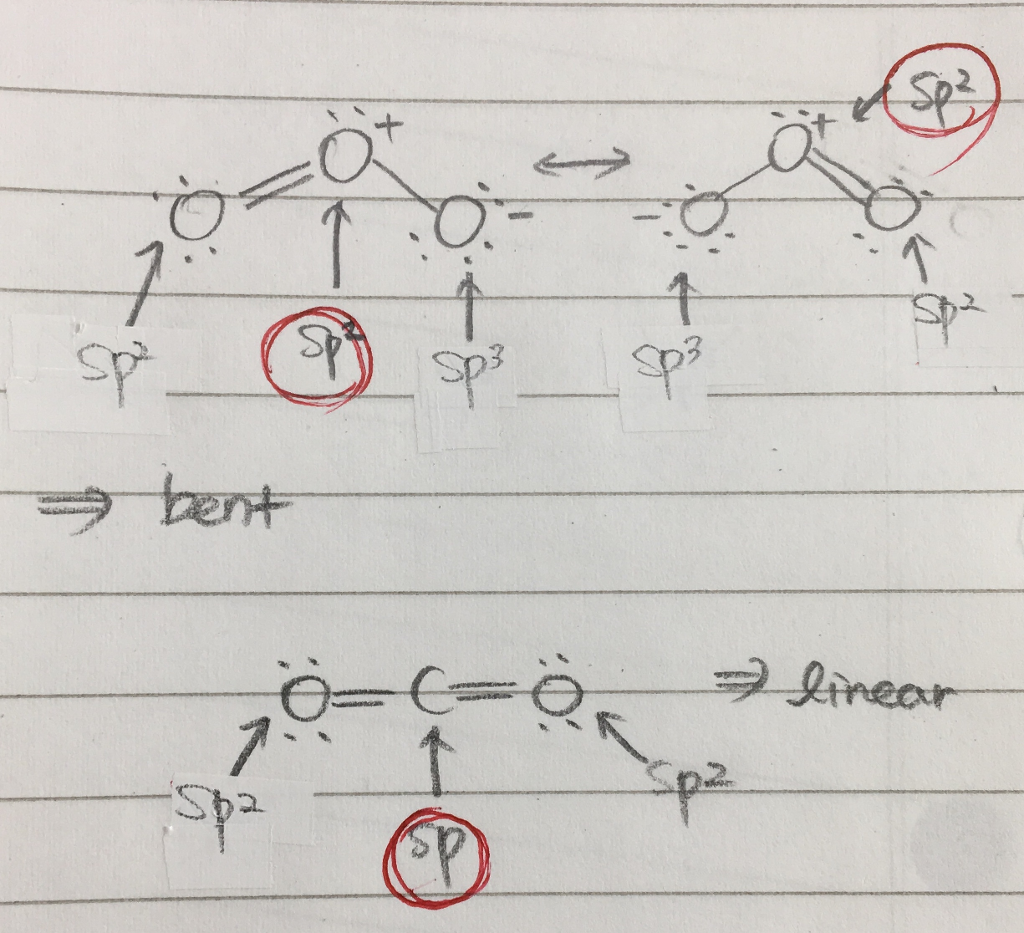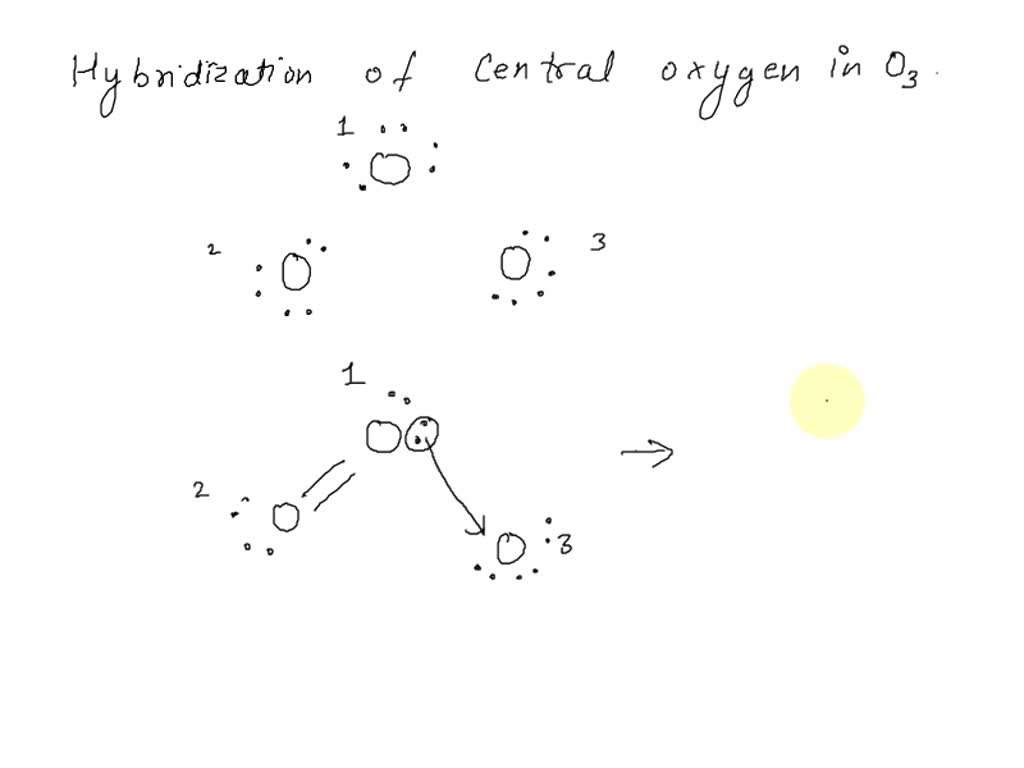
SOLVED: What is the state of hybridization of the central O atom in O3 ? Describe the bonding in O3 in terms of delocalized molecular orbitals.
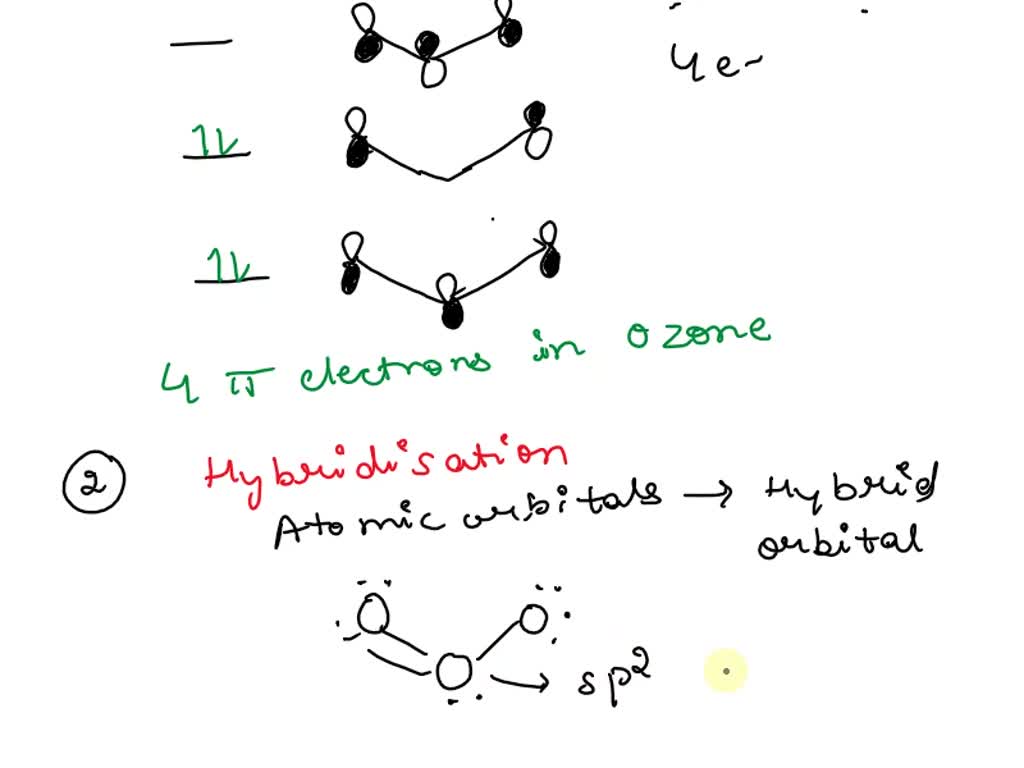
SOLVED: a) Ozone O3, has a three-atom T system. How many T electrons are there in ozone? Add the electrons to the energy level diagram (arrows) side vicw b) The three oxygen
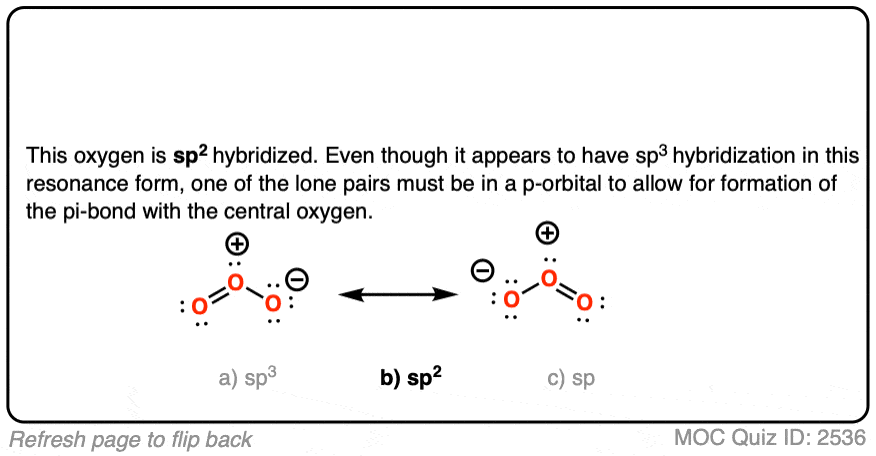
![High School Chem]: Is this the correct Lewis Structure for ozone (O3)? What is the formal charge of each oxygen? What is the hybridization of the middle oxygen? : r/HomeworkHelp High School Chem]: Is this the correct Lewis Structure for ozone (O3)? What is the formal charge of each oxygen? What is the hybridization of the middle oxygen? : r/HomeworkHelp](https://i.redd.it/pzmn9pdp2io41.jpg)
High School Chem]: Is this the correct Lewis Structure for ozone (O3)? What is the formal charge of each oxygen? What is the hybridization of the middle oxygen? : r/HomeworkHelp

Chapter 10 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals Atoms are bonded together by electrons, but what is a bond? A. - ppt download
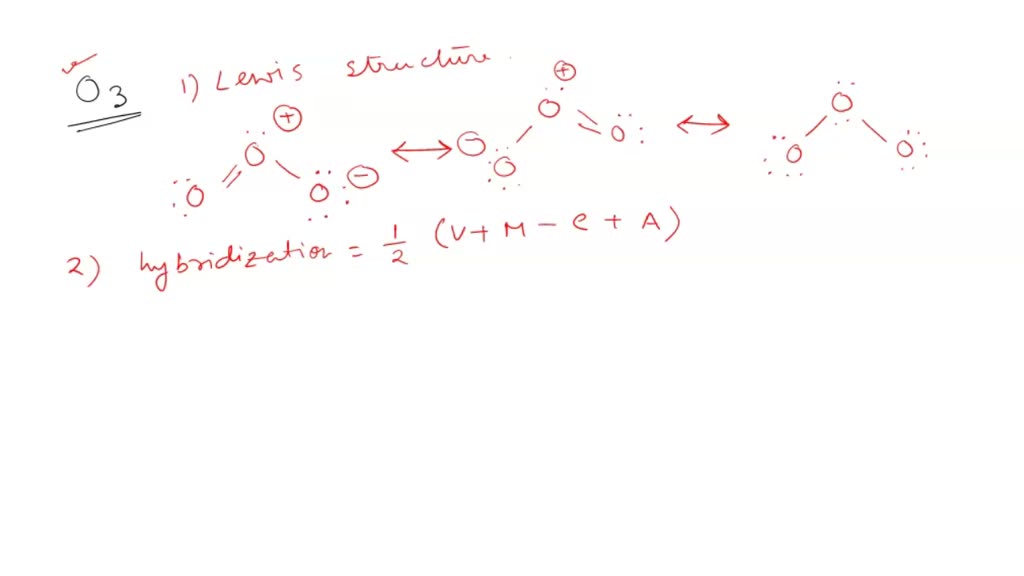
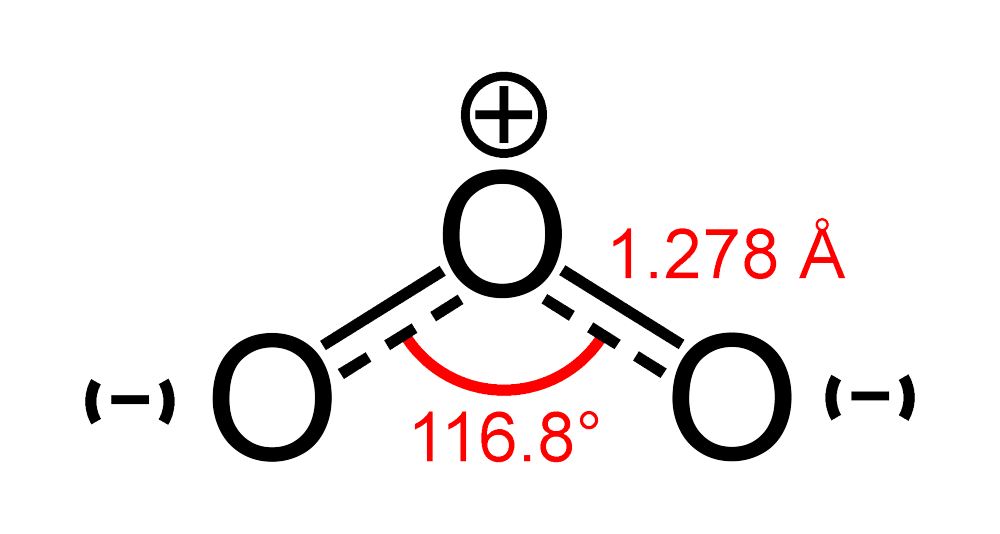
SOLVED: Ozone O3 is a compound with 3 oxygen atoms. Draw the Lewis structure and then determine the hybridization on the central oxygen atom so that you may state which orbital would
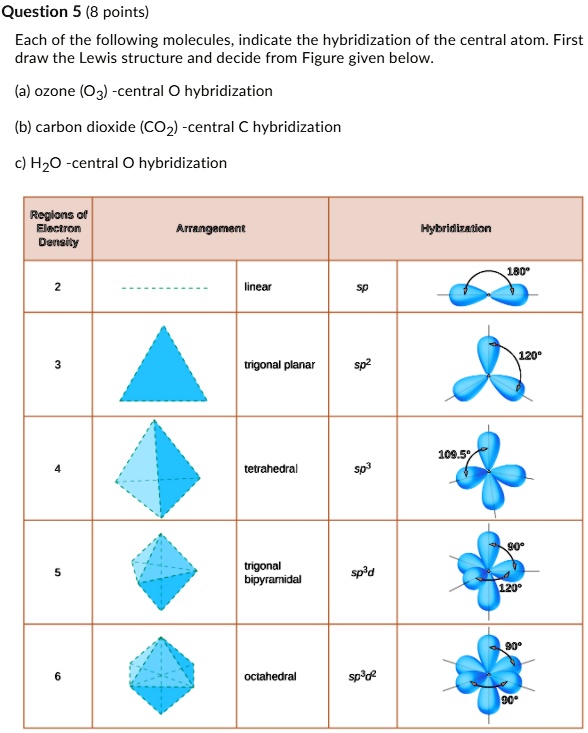
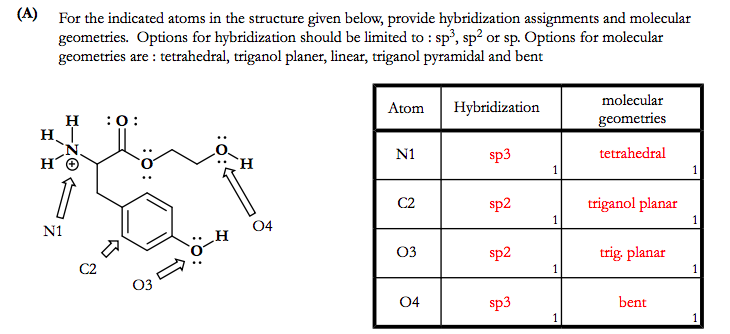
SOLVED: Question 5 (8 points) Each of the following molecules indicates the hybridization of the central atom. First, draw the Lewis structure and decide from the figure given below: (a) ozone (O3) -